It’s not every day that the periodic table of the elements is featured in a comic book, let alone a Batman comic.
This time, the once-in-a-rare while event happen in Batman #45. In the storyline, the now Mohawk-wearing (former Commissioner) Jim Gordon is now Batman, or rather “Batman,” a corporate-sponsored hero to fill the void left by the real Batman, whom Gotham assumes to be dead after an epic battle with the Joker in “Endgame.”
He’s not, really, of course…Bruce Wayne has just had his…um…well, maybe you should read the series to catch up on the Bruce stuff. We’re here to talk about elements. The storyline that the above-mentioned periodic table shows up in is called “Superheavy,” and began in issue #41.
In issue #45, Gordon meets up with Geri Powers (owner of Gotham-based Powers Industry which acquired Wayne Enterprises following Wayne’s disappearance…after that Joker fight mentioned earlier) at the Powers Industry supercollider/science museum. Powers updates Gordon on what Powers Industry has been doing in regards to synthesizing new elements to fill in the periodic table. Giving Gordon a slight Nuclear Chemistry 101 lecture, Powers explains that above elements in the 80s (Bismuth, Polonium, Astatine and up through Uranium), the atoms of the elements are too big and just start falling apart – a process we call nuclear radiation.
Powers also mentioned a Sea of Instability – a stretch of elements where the atoms are too large to hold together and fall apart virtually instantly. This “sea” can be crossed to reach an “Island of Stability” – a place on the expanded periodic table which is home to stable new elements whose combination of protons and neutrons in their nucleus allow for them to hold together for longer periods of time, perhaps long enough to be used in products or combined with other elements.
Powers then reveals to Gordon that Powers Industry has done it – they have crossed the sea of instability and were able to create four new elements, with the newest being element number 206. She then shows him a Bat-symbol made of this new element 206 (quickly nicknamed Batmanium by Gordon), which Powers says weights two tons and is also a superconductor.
 They get to talking about other things, and don’t return to the two ton Bat-symbol thing (a superheavy Batarang? An emblem for the front of a new Batmobile that won’t mind the weight of another car on its grill?) for the remainder of the issue, and maybe the storyline, although it seems to be the arc’s namesake, as element 206 would be, by definition, superheavy.
They get to talking about other things, and don’t return to the two ton Bat-symbol thing (a superheavy Batarang? An emblem for the front of a new Batmobile that won’t mind the weight of another car on its grill?) for the remainder of the issue, and maybe the storyline, although it seems to be the arc’s namesake, as element 206 would be, by definition, superheavy.
Okay – we’ve got a lot to dig into here when it comes to science, so let’s get started.
First up, kudos to the issue’s writer, Scott Snyder for having Powers’ mini course on nuclear chemistry be spot-on, and artists Greg Capullo and Danny Miki for not scrimping too much on the depiction of the periodic table. So many boxes…sooo many boxes…
But let’s get to the science – and we’ll start by setting this table.
Who Gets a Seat at the Periodic Table?
You probably remember this from being on the wall of one or many science classes you’ve had over the years. Depending on how awesome your science teacher was, you may remember it fondly. Or…well, let’s just not talk about that now, okay?
We’re just going to skim the surface here, but if this gives an itch, by all means scratch it. Check out ptable.com for starters, and then check the videos and resources at the end of this article for more periodic table goodness.
 First things first – the Periodic Table is a graphic organizer. We say it’s the best in the world, but we’re biased. Everything is where it is for a reason. No element is just placed somewhere for convenience’s sake. There are seven rows (periods) and 18 columns (groups or families). The elements are identified by their chemical (or elemental) symbol, and are arranged by increasing atomic number. Things kick off with Hydrogen at number 1 to (currently) Livermorium at 116. The recently (officially) added 117 and 118, along with 113 and 115 have name designations that are just placeholders that are there until the International Union of Pure and Applied Chemistry (IUPAC) approves of the names submitted by the teams that discovered the elements.
First things first – the Periodic Table is a graphic organizer. We say it’s the best in the world, but we’re biased. Everything is where it is for a reason. No element is just placed somewhere for convenience’s sake. There are seven rows (periods) and 18 columns (groups or families). The elements are identified by their chemical (or elemental) symbol, and are arranged by increasing atomic number. Things kick off with Hydrogen at number 1 to (currently) Livermorium at 116. The recently (officially) added 117 and 118, along with 113 and 115 have name designations that are just placeholders that are there until the International Union of Pure and Applied Chemistry (IUPAC) approves of the names submitted by the teams that discovered the elements.
The numbers by which the elements are arranged are their atomic numbers, and represent the number of protons in the nucleus of an atom of that element. Neutrons are also found in the nuclei of elements’ atoms, and those can be roughly calculated by taking that decimal number (the average atomic mass), rounding it to the nearest whole number and subtracting the atomic number from it.
Something very important to where we’re headed is that Uranium, element number 92 (92 protons and, on average 146 neutrons) is the last naturally occurring element. In other words, it’s the last element that can be found in earth’s crust (although you may get argument that it actually runs through to Plutonium, at 94). Everything from Neptunium (93) through 118 (which would include the hypothetical Batmanium, element 206) has to be made.
Seriously – there is so much cool stuff about the Periodic Table that we just can’t get into here, otherwise we’d be here forever…do yourself a favor and dig into it now and again – there’s history, adventure, politics, war, intrigue and science all wound up in that collection of boxes. A great, and extremely accessible starting point is Sam Kean’s The Disappearing Spoon, which is available in all formats, everywhere.
Making the New
Obviously, our reason for being here, element 206 is a far way from 92. How do we get there? That’s simple – you have to make new elements. And for that, we turn to the father of the superheavy elements, Dr. Glen Seaborg.
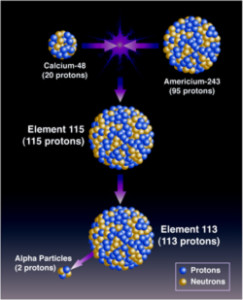
 Working at the University of California, Berkeley, Seaborg (along with others) figured out the easiest way to make new, superheavy elements was to, as Powers explained, shoot small atoms at bigger atoms, and hope that the math works out, and your final product is stable.
Working at the University of California, Berkeley, Seaborg (along with others) figured out the easiest way to make new, superheavy elements was to, as Powers explained, shoot small atoms at bigger atoms, and hope that the math works out, and your final product is stable.
Let’s jump ahead in time and talk about the recently confirmed Element 117. To create it, a target made of 22 milligrams of Berkelium (one of Seaborg’s creations) was placed in a type of particle accelerator called a cyclotron. Calcium atoms with a positive electric charge, (called calcium ions – atoms stripped of electrons as Powers told Gordon), are accelerated and fired at the Berkelium target for 150 days. There are numerous “hits” as a result, with the vast majority of the collisions between Calcium and Berkelium smashing into smaller bits and pieces. With roughly one hit in a billion, though – you get magic. Berkelium’s atomic number is 97, Calcium is 20 – 97 + 20 = 117.
But that magic is short-lived. The new atom of Element 117 gets separated out and detected. During the 150 days the experiment was run, six atoms of Element 117 were created and detected. 117 starts to break down within milliseconds of its creation, losing alpha particles and ultimately fissioning (splitting) into its fission products.
Very generally speaking, that’s the process – shoot something bigger with something smaller, and hope that in one of the atomic collisions, everything sticks together and is stable…for at least a fraction of a fraction of a second.
But – just like bullets and guns, you can run into problems when you’re talking about ions and particle accelerators.
(Oh, and one quick shout-out: in the premiere episode of The X-Files miniseries, it was explained to Fox Mulder that element 115 was involved in the warp drive of the UFO that he saw. Cool.)
Elements through 118 have been “discovered,” that is, synthesized in labs through the use of Calcium “bullets.” The problem is, that approach can’t be used as effectively once you get past 118, which is where researchers are now setting their sights. Isotopes of other elements are possible candidates for new projectiles to fling at targets made of larger atoms – such as titanim-50, ion-58, nickel-64 and chromium-54. But – larger bullets generally need larger guns to get them up to the speed at which they do what you want them to do. Bigger and more powerful particle accelerators will be needed.
Projects to create elements 119 and 120 are already underway, but again, when thinking about the Periodic Table, researchers will be getting into terra incognita – Period 8 (the eighth row) of the table. Following the structure of atoms that the table reflects, the electrons of many of Period 8’s elements will be located in the 8th primary energy level.
Also – as elements have gained the higher ground in the atomic number game, instability has kept pace, which larger and larger elements being stable long enough to be detected. But – Seaborg suggested what Powers explained to Gordon in Batman – there may be “Islands of Stability.” These “Islands” would be regions of the Periodic Table where the protons and neutrons of the nucleus are arranged in specific energy layers that allow them to…well, exist. For longer than a few milliseconds. Maybe even long enough and stable enough to produce more than a few atoms that disappear after a few milliseconds. Predictions for the Island of Stability range from the recently discovered elements of the 117 – 118 being the “shore” of the island, with elements around 126 forming the bulk of the stable elements of the island. Elements with the “magic number” of protons and neutrons could possibly hold together like normal, earth-inhabiting elements.
The existence of a second Island of Stability has been suggested, this one based around an isotope of Element 164 (164 protons and 318 neutrons), but to get there – bigger, faster and stronger particle accelerators are going to be needed.
And after that? Hey, even getting this high is pretty weird, what with your nuclear particles acting more like waves than actual particles and what not. But is there an upper range to the possible atomic structures in our universe with its particular atomic constants?
Maybe.
Renowned physicist Richard Feynman said that nothing more would be possible after Element 137, due to relativity. Citing Einstein, Feynman pointed out that given the attraction between the negative electrons and the positive protons in the nucleus, electrons would have to move faster than the speed of light in order to circle the nucleus, rather than crash into it.
Feynman’s calculations have been revised since his original prediction, with the relativistic upper limit now being around 173, although there are some theories that it’s possible for elements to have even more protons in their nucleus. Above 173, some predictions have atoms being able to cause electrons to materialize from empty space.
It’s easy to think that these superheavy elements are completely artificial, that is, things we’ve created just so we can study them. But by most theories, superheavy elements, with higher atomic numbers than those that can be found on earth may exist on their own, perhaps in environments that can support their creation and existence, such as the centers of dense neutron stars. Or black holes. Who knows what kind of matter will be found in and around black holes?
But overall, the consensus is pretty clear among physicists and chemists – we don’t know how high the Periodic Table will eventually go.
Batmanium!
 So – as always, we’re not here to rain on parades and say things in pop culture can’t exist. If you’ve made it this far, you’ve probably learned a thing or two about superheavy elements and the Periodic Table, and for that, we’ve met our goal.
So – as always, we’re not here to rain on parades and say things in pop culture can’t exist. If you’ve made it this far, you’ve probably learned a thing or two about superheavy elements and the Periodic Table, and for that, we’ve met our goal.
But what about the Batmanium, the element with 206 protons and who knows how many neutrons? As Powers tells Gordon, her team did it – found a new Island of Stability and created four new atoms, the newest being 206 (so we’re going with the idea that they created 203, 204, 205 and 206, and those elements make up the bulk of the new Island).
As Powers says, the Bat-symbol her team made of the element weighs two tons. And is a superconductor. The first characteristic – high mass? Sure, we’ll buy that. As you keep packing more and more protons and neutrons into a nucleus, you’re mass and therefore your weight increases as well. The volume of the Bat-symbol is pretty hard to estimate from the artwork, but if we could, we could make some further estimates about the number of neutrons in an atom of Element 206.
What about superconductivity? In virtually all instances of superconductivity in our world, the lack of internal resistance occurs when the materials are cooled below a critical temperature. The Bat-symbol looked to be at room temperature, so hey – maybe materials made from elements on that Island of Stability are truly weird and freaky.
In terms of the Periodic Table and element 206 – which row and column it would be in…there’s lot of work to do before that could even be guessed at. As you can probably guess though – science is already on it.
Batman #45 is available at your local comic shop or here at Comixology.