Nth Metal. Promethium. Dionesium. Electrum. Batman-ium.
These are the five metals – the “five impossibly heavy metals” or, if you prefer, the five divine metals of the DC Universe. They’re also the five metals that Batman was treated with in Dark Knights: Metal that allowed him to be the gateway that allowed the dark god, Barbatos and his Dark Knights into the DC Universe.
As it wraps up this week, Dark Knights: Metal has been an epic tour through the fantastic science of the DC Universe, stretching from the beginning of the cosmos to the dark multiverse and its dead earths and universes, with many stops in between that touched on Plastic Man’s hyper-elastic molecules, energy “off of the electromagnetic spectrum,” dark lightning, dark suns, and a cosmology larger than either the vast multiverse that existed before the Crisis on Infinite Earths, or the more restrained “52” reboot, which brought the number of multiple universes of the DCU down from infinity down to a more manageable 52.
The event series has reinvigorated DC’s wild science side – in a big way.
But back to the five elements that Batman was treated with – a process called “The Mantling.” We’ve already talked about the origins of “Batmanium” (the name seems to be sticking, yes) and the science behind it, and the other four are a mix of historic DC elements (plot/story and…elements), along with some new introduced by Metal writer Scott Snyder during his run on the Batman series.
The new elements brought together in Metal – and any “new elements” brought up in pop culture (Gravitonium over in Agents of SHIELD, Wolverine’s Adamantium, Wakanda’s Vibranium, Star Trek’s Dilithium, and Avatar’s Unobtanium to name just a few) are often brought up in either my chemistry or physics classes. Students will ask if such elements exist, why they don’t, and then – hopefully – take a look at the Periodic Table of the Elements on the wall, and things really get going. It’s become such a common occurrence, that I usually bring them up myself at the start of our work with the periodic table.
Occasionally, as we talk about the “open endedness” of the periodic table and where it currently “ends” (Oganesson – 118) I may get a version of “you never know…” in regards to that student’s favorite fictional element.
And then we’re off to the races. Now the learning is going to happen, because that students is invested in 1) their favorite fictional element and explaining its powers, and 2) proving the teacher wrong.
So let’s talk about the science of superheavy elements, and let’s use Dark Knight: Metal’s five as our examples.
But we’re going to have to do a little reviewing of our chemistry first…
Wait – come back! It’ll be painless. Promise.
And no test at the end.
What Are Atoms Made Of Again?
Let’s go back to chemistry class for a few minutes. Atoms are made of three sub-atomic particles (and we’ll just stop at those three, although we’ll talk about quarks briefly later on): protons, neutrons and electrons. We’re going to skip out on electrons for a little while – but they’ll be back. All protons have the same amount of positive charge, a mass of 1 atomic mass unit and are located in the nucleus of the atom, while neutrons have no charge, a mass of 1 atomic mass unit, and are also located in the nucleus. The nucleus itself is very small compared to the rest of the atom – only taking up __% of the atom’s total volume.
Bring a positive charge near another positive charge, and they repel one another. The closer the positive charges are to each other, the stronger that repulsive force. These forces of repulsion, and their opposite forces of attraction are called Coulombic forces, named after Charles Augustine Coulomb who proved that the forces between two charged particles was proportional to the inverse square of the distance between them. Small distances give strong forces.
So – in something as small as a nucleus, all of the protons of the atom would rather just shoot off somewhere-anywhere-else. Sure, you could push and squeeze really hard to make them stay together, but that takes a lot of pressure – the kind that you find in the hearts of stars. Not really what we’re looking for. The force that holds the nuclei of atoms together has to be stronger than the electric/Coulombic force trying to push them apart and it is – and it’s even named so you can conveniently remember that fact – it’s called the strong nuclear force.
The strong nuclear force acts over an extremely small distance, which in part explains why an atom’s nucleus is so small – and also can be seen as putting an upper cap, in a way, on just how big a nucleus can get. And that’s where we’re headed.
Metals, Heavy Metals and Superheavy Metals
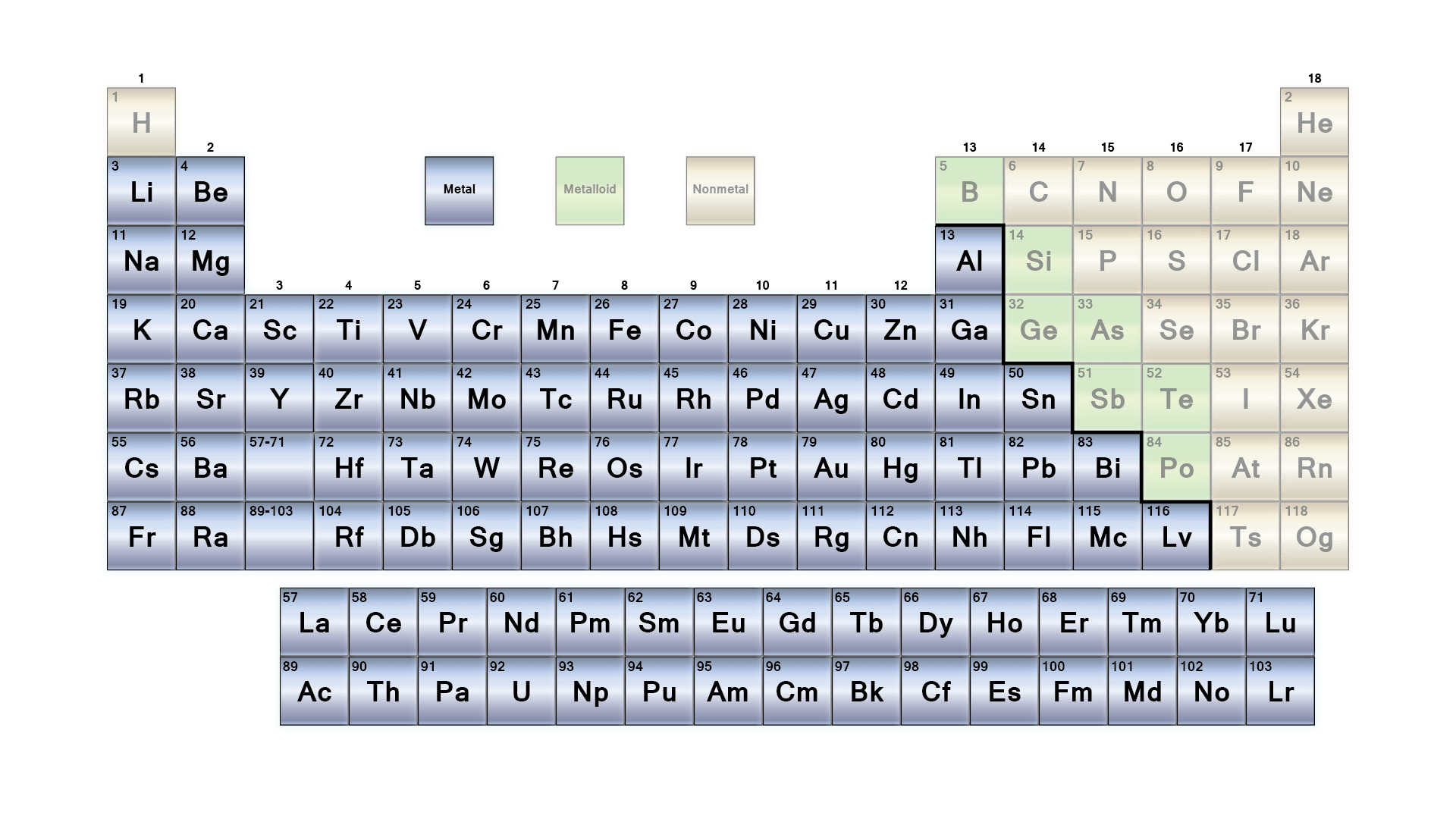
Let’s take a look at the periodic table,, highlight only the metals.
Yeah – there are a lot. About 91 of the 118 are metals. The remainder are nonmetals (thinks like sulfur, carbon and oxygen) and metalloids (silicon and its buddies). All metals have similar characteristics – such as ductility, malleability, and electrical conductivity. Of the metals, they can be further classified into heavy metals and superheavy metals based on a few kind of hazy criteria. For example, if you’re a chemist, you might say heavy metals all have the same behaviors in chemical reactions, but if you’re a physicist, you may have a bright line in regards to atomic numbers where the “metals” end and the “heavy metals” begin on the periodic table. Sometimes, a density value of 5 g/cm^3 is used for classification, but even that isn’t universally agreed upon.
Maybe it’s better to just think of some examples. Light metals are things like lithium, aluminum, magnesium and sodium. Heavy metals include many that are common in everyday life, such as copper, iron, tin, gold and the bismuth that makes Pepto Bismol work so well. So what about superheavies?
Again – the definition of what makes up a superheavy element/superheavy metal depends largely on who you ask. Sometimes “superheavy” is used as a shorthand for the transuranic elements, elements with higher atomic numbers than uranium (92), while others will tell you that transuranics end at 99, and any element with an atomic number higher than 100 is a superheavy. Regardless, the superheavy elements are well-named. As far as elements go, they’re the big ones. And making them isn’t easy.
An Atom-Sized Bullet
Elements #1 – #92 occur naturally on earth, so if you want en element with an atomic number higher than 92, you have to make it. The how is simple in theory – smash a smaller atom into a bigger one. But there are problems.
Nuclei of atoms are positive, remember? And positive things don’t like to be near other positive things, so they’re going to repel if you just try to push them closer together and kiss. As mentioned earlier, you’re going to have to overcome Coulombic forces, and to do that, you’re going to need a lot of energy. Not only that, you’re going to need a lot of luck, because not every shot is going to hit the target.
In terms of energy, you need to accelerate the “bullet” particles (in a particle accelerator) to speeds of about 10% the speed of light before shooting it at your target. And a lot goes in to picking the “bullet” and target atoms – you want something that has a large number of neutrons to help hold things together, but you can’t use something that large, because even though the “bullets” are atoms, it takes more energy to accelerate heavier “bullets” than it does lighter ones.
For example – to make an atom of Fermium-244 (Fermium with 100 protons and 144 neutrons), an atom of Argon-40 (18 protons and 22 neutrons) is fired at Lead-208 (82 protons and 126 neutrons). When everything works just right, the speed of the “bullet” is enough to overcome the electrostatic repulsion between the Argon and Lead nuclei and, in this case, the nuclei fuse, with four neutrons lost in the process. That’s one atom.
Batman sidebar – we’ve talked previously about how Batmanium was created by Geri Powers and the particle accelerator under Gotham City, and more about how this particle accelerator stuff works. You should check it out.
Most of the time though, you don’t even get that one atom. The stuff falls apart. Fermium-244 breaks apart into other stuff in 4 milliseconds, or 0.004 of a second. Still – that counts as an element, so it gets its own place on the periodic table.
Go for bigger atoms, and it’s much tougher for the strong force to hold the nucleus together (more protons = more trouble). Current technology uses Calcium-48 (20 protons, 28 neutrons) as its “bullet,” and with element #118, Oganesson, we’ve reached the end of what we can do with Calcium “bullets.”
And those are the two big problems with the superheavy elements – making them and falling apart. In fact, around atomic numbers #83 – #84 (bismuth and polonium), atomic nuclei just get so big they’re naturally unstable and fall apart – something we call nuclear radiation. All of the superheavys in our world are radioactive, and extremely short-lived.
Islands in a Turbulent Sea
Projects to create elements 119 and 120 are already underway, but again, when thinking about the periodic table, researchers will be getting into terra incognita – Period 8 (the eighth row) of the table. Following the structure of atoms that the table reflects, the electrons of many of period 8’s elements might be located in the 8th primary energy level – which doesn’t exist as far as modern chemistry understands it.
Also – as elements have gained the higher ground in the atomic number game, instability has kept pace, which larger and larger elements being stable long enough to be detected. But – there may be “Islands of Stability.” These “Islands” would be regions of the Periodic Table where the protons and neutrons of the nucleus are arranged in specific energy layers that allow them to…well, exist. For longer than a few milliseconds. Maybe even long enough and stable enough to produce more than a few atoms that disappear after a few milliseconds. Predictions for the Island of Stability range from the recently discovered elements of the 117 – 118 being the “shore” of the island, with elements around 126 forming the bulk of the stable elements of the island. Elements with the “magic number” of protons and neutrons could possibly hold together like normal, earth-inhabiting elements.
The existence of a second Island of Stability has been suggested, this one based around an isotope of Element 164 (164 protons and 318 neutrons), but to get there – bigger, faster and stronger particle accelerators are going to be needed.
And after that? Hey, even getting this high is pretty weird, what with your nuclear particles acting more like waves than actual particles and what not. But is there an upper range to the possible atomic structures in our universe with its particular atomic constants?
Probably.
Physicist Richard Feynman said that nothing more would be possible after Element 137, due to relativity. Citing Einstein, Feynman pointed out that given the attraction between the negative electrons and the positive protons in the nucleus, electrons would have to move faster than the speed of light in order to circle the nucleus, rather than crash into it.
Feynman’s calculations have been revised since his original prediction, with the relativistic upper limit now being around 173, although there are some theories that it’s possible for elements to have even more protons in their nucleus if a spherical shape is not assumed. Above 173, some predictions have atoms being able to cause electrons to materialize from empty space.
Superheavy Properties
One last thing that you may remember from chemistry class is that elements in the columns (groups or families) have the same characteristics – or act the same in chemical reactions – and that’s due to them having the same electron configuration in their outermost layer of electrons (extra credit for whoever was out there muttering “valence shell.’)
That said, the properties of the superheavy elements should follow along, right? Technically speaking, element 118, Oganesson, should behave like the rest of group 18, the Noble Gases. Thing is, it doesn’t and they don’t. For example, element 114, Flerovium, acts something like a metal and a gas despite the fact that it’s not near any gas families. And Oganesson gets even weirder.
As mentioned earlier, as atomic nuclei get larger, the electrons get more energetic – moving faster and faster, until their headed toward the speed of light. This high energy keeps the electrons of Oganesson out of specific rings and shells, and more into just a blob. What this means for the element’s properties and reactivity is up in the air. There are some predictions, but more Oganesson needs to be created to test the theories out. One of the phrases bouncing around with elements 118 and the (probably within 5 years or so) soon to be discovered 119 and 120 is that we’ve pushed the periodic table to the end of periodicity. That means that around 118, the columns are no longer distinctive “families” whose members all behave the same way.
I Just Came Here to Read About Batman, Man…
(speculation ahead)
Okay, okay – I came here to talk about Batman and the metals of Dark Knights: Metal, so let’s get back to Nth Metal, Promethium, Dionesium, Electrum, and Batmanium.
Batmanium we know – that’s element 206, and it’s superheavy and a superconductor. Reality-wise, it’s off the charts of even being predicted. In our world, with our understanding of physics and chemistry, there’s no way element 206 would be stable (non-radioactive). Likewise with the remainder of the metals, if they are in fact, elemental metals. Remember – everything above Polonium is unstable and thus radioactive. Promethium, Dionesium, and Electrum are named like elements – the -ium at the end of their name is a Latin suffix meaning “derived from” – Calcium is “from calyx,” Potassium is “from potassa” (potash), and the naming convention has stuck. So if they’re elements, they’re superheavies, and radioactive – which is always a good category to push “unexplained powers” into, and as with Oganesson, they don’t have to follow any pre-established properties.
If they’re superheavy elements, where could they have come from – originally? Batmanium was manufactured – a part of a millenia-old plan based on the idea that someday, someone would create element 206, and somehow Bruce Wayne would encounter it. But the others? Where could they have come from?
Since this is comic book science we’re talking about, why not space? It worked for Vibranium in Black Panther, right? Could superheavy elements be made in space? Possibly. Virtually all of the elements are formed from fusion in the center of stars. As stars get older, they produce higher and higher element numbers until they get to Iron. After that – it’s bad times for the star, and if it’s big enough, it may explode, creating elements with even higher atomic numbers that iron – all the way through to uranium. Could some weird star’s life cycle have created the other elements? It can’t be ruled out, but again, stability is the thing.
Even if a supernova produce conditions that were right for creating tons of, say, element 230, our understanding of physics and chemistry has it being very radioactive and falling apart. Could it be on another, higher, island of stability? As we understand it, even in the center of an island of stability, the element would fall apart in a thousand years. If it’s traveling through space, a thousand years doesn’t allow for much distance. There is a push in Russian physics to look at meteorites to see if they’ve had superheavy elements pass through them, leaving trails in a mineral common to space rocks, but even the most optimistic researcher isn’t thinking that any quantity of superheavies will be found in space.
But again – who knows? We used to think we had a good handle on what kind of exoplanets could exist, and results from Kepler and other observatories have shown “impossible” exoplanets that defy our theories and call for a new or better understanding of what we thought we knew.
What about Promethium, Dionesium and Electrum being some kind of “hidden” elements – somehow “hidden” within the tiles of the periodic table itself in defiance of everything we thought we knew? No – at least as we understand the science. Atoms are different from one another due to their number of protons (their atomic number), and as the periodic table shows, we’ve got 1-118 filled. There’s no “element 6.5” as we understand it for a lot of reasons.
But – in a totally theoretical sense, what if a proton’s not a proton as we know it? Perhaps the protons of Promethium, Dionesium and Electrum are made from different “pieces” than ours. Protons and neutrons are each made of three quarks. Protons: Up, Up, Down quarks and Neutrons: Down, Down, Up quarks. There are four other types of quarks: Charm, Strange, Top and Bottom. These quarks are heavier than Up and Down and have different properties, but who’s to say? Maybe in an alternate universe (which the DCU certainly is in), different quarks come together to form their “protons” and “neutrons.” Research seems to suggest that while our protons and neutrons have permanent quarks (Up, Down), the four others will flit in and out, subtly altering the proton or neutron properties. What if, in an alternate universe or another part of our universe where there might be slightly different physics the permanent quarks are different and the Up and Down flit in and out?
Could all of that create new elements, or at least elements that are superheavy, but are further down the line on the periodic table? Maybe.
What about Nth Metal? I’m betting on alloy – a combination of metals like brass (copper and zinc) or bronze (copper and tin). Nth Metal (or “ninth metal”) is occasionally referred to as an alloy by characters and fans, so the line is pretty blurred. It’s certainly a metal, given its many uses, but as for its properties and their physics-defying abilities…it’s safe to go with a combination of two or more metals, perhaps one with exotic properties.
Blending metals where one part is radioactive isn’t unheard of, even on our world. The impressively named Mag-Thor is an alloy of magnesium and the radioactive thorium that’s sometimes used in missiles and other (mostly military) applications. Alloys like Mag-Thor keep some of the properties of their components while often showing new ones as well. Nth Metal – it’s activity and properties tend to suggest that you’ve got a mix on your hands.
As for Nth metal’s headliner property – the ability to defy gravity? Yeah – that one’s going to take a while to sort out.
And all the metals together, as we saw in Metal #2 can open passageways between different universes. Whoa. That’s so much…um…yeah…by our understanding of physics and cosmology, that’s a lot of energy.
So…Where Are We Now?
Hopefully, knowing a little more chemistry than when you came in, and maybe – just maybe, interested enough in it to keep digging and keep learning. Man-made elements are fascinating business, and there’s just something inherently goose-bumpy about electrons that are traveling nearly at the speed of light, or matter made of different ingredients. This is fun stuff.
As for Metal? It’s epic. It’s been a wild ride. And while creators Scott Snyder and Greg Capullo may not have set out to revitalize science in the DC Universe, they kinda sorta did give it a kick and maybe helped to organize things a little bit. I’m not ever wanting the DC Universe to enforce a strict science policy in their universe, but it is nice to see some of the science treated with a bit of forethought and actually having it play a role in the story.
And who knows, 30 years from now, maybe someone will be accepting an award for chemistry or physics for some breakthrough in material science, and they’ll start their speech with, “You know…back around 2017 or 2018, there was this comic book about Batman and some metals that got me thinking…I guess I just never stopped thinking about it.”
Inspiration has to start somewhere…