In last week’s episode of The Flash (you can still catch it on The CW’s website here), the story reached to a point where the Flash (Barry Allen) had to rescue Professor Martin Stein from General Wade Eiling and reunite him with Ronnie Raymond (so the two could later form Firestorm). Eiling’s troops attacked, shooting a rocket-fired projectile at the Flash. Although he grabbed the missile in mid-air, it exploded while the Flash was holding it, coating him with a white chemical, which was sizzling. Something bad was going on.
As the Flash told Cisco and Professor Harrison Wells (the Flash’s mentor and radio-connected guardian angel, respectively), Eiling’s troops hit him with a chemical. Cisco pointed out that the Flash’s suit had some “serious pH numbers” going on, and that the burning chemical was “weaponized phosphorous.”
“But you can’t burn in a vacuum,” Professor Wells said, “So you need to create one…run Barry, run!”
And Barry ran off…
We can’t speak for what the show’s version of a “weaponized phosphorous” might be, but in the real world, we’ve got a pretty good weaponized phosphorous – it’s called white phosphorous.
So – what is this stuff?
Winding the clock back a little, phosphorous, the element, was first isolated in 1669 by Hennig Brand, a German physician and alchemist. Like all good alchemists, a huger for gold inspired Brand in his pursuit. Unlike his contemporaries, Brand was also fascinated by water. Putting his two interests together, the alchemist turned his attention to…urine. Don’t judge. It is a gold liquid, after all. Just not…liquid gold.
In a series of what must have been horrific smelling experiments, Brand started by boiling urine down to thick syrup and ultimately recovered viscous liquid that burst into flames when it came in contact with air. Even after its flames went out the substance continued to glow. Brand named it phosphorous from the Greek for “light-bearer” or “light-bearing.” Check out the video below for more:
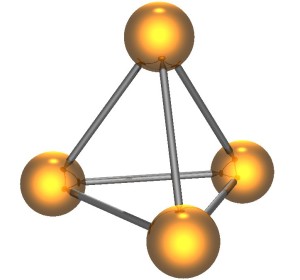
White phosphorous (or WP) is an allotrope of phosphorous. Allotropes are forms of an element with their own bonding between atoms. Think of charcoal and diamonds. Both are carbon, but the atoms are bound together differently. One you cook food over, the other you make jewelry out of.
WP is made up of four phosphorous atoms bonded together in a tetrahedral shape. Each of the four phosphorous atoms is bonded to three others, and the bonds are strained. They’re ready to break and release energy. And they will.
By the way, a more stable allotrope of phosphorous is red phosphorous, which is found in matches. As white phosphorous is exposed to air, more and more of it turns into red phosphorous. White phosphorous with a small amount of red in it is no longer pure white. The (formerly) WP takes on a yellowish tint, and is often referred to a “yellow phosphorous,” which is not a true allotrope, but rather a mixture of white and red. Other forms include black and violet.
White phosphorous is the least stable allotrope of phosphorous and ignites spontaneously in the presence of oxygen at 93°F (34°C). The chemical reaction looks like this:
P4 + 5O2 -> 2P2O5 (along with other phosphorous oxides)
The P2O5 is hygroscopic (takes up moisture) and then creates phosphoric acid like this:
2P2O5 + 6H2O -> 4H3PO4 (along with other versions of phosphoric acid).
In other words, as the WP burns, the diphosphorous pentoxide (P2O5) hooks up with the moisture in the air to form phosphoric acid, which can coat objects or be created in the moisture-rich airways of anyone unlucky enough to be near the burning white phosphorous.
Check out white phosphorous burning from Periodic Table of Videos:
White phosphorous burns at around 5000°F (2760°C) and will continue burning as long as it can reach air. It’s for this reason that water isn’t your best bet to put it out. As soon as the water evaporates, the burning WP will re-ignite. Not only that, the water you’re using may superheat and mix with some of the phosphorous oxides to produce phosphine gas which is highly poisonous. Fire suppressing and retardant foams wouldn’t work well either, unless the bubbles making the foam contain no oxygen. Best bets for putting out white phosphate – smother with cold water (a lot), wet sand or soil (think mud), and copper salts, such as copper sulfate.
Touching back on what Wells said to the Flash, a complete vacuum with no oxygen would be an effective means to put out a WP fire. Or you could smother the burning phosphorous with any non-oxygen gas, like nitrogen or argon.
Another thing about burning white phosphorous is that it produces a lot of smoke – instantly. Thanks to this property, WP has long been used in smoke grenades or other projectiles where large amounts of dense, choking smoke are needed quickly.
As you can probably guess, none of these properties of WP make it something that you want to have anywhere near your body. If it touches your skin, it will most likely stick, and burn – probably to the bone. Additionally, contact brings in the very real danger of phosphorous toxicity, which can lead to kidney, liver and heart damage, multiple organ failure, and death. Smaller pieces will continue to smolder, even when deep within tissue, and continue to produce phosphoric acid.
It probably goes without saying that WP is often used as a weapon. Introduced into warfare by the British Army in 1916, WP has seen action in every major (and many minor) armed conflict around the world. Most recently, it has been used extensively in the Middle East, by forces from all sides of every conflict, apparently against armed forces as well as civilians with bombs designed to explode over targets, showering the soldiers and civilians below with burning white phosphorous. The United States military (which nicknamed it “Willy Pete” during Vietnam) still uses white phosphorous in many forms, from smoke grenades to Mark-77 bombs.
There are no specific treaties addressing the use of white phosphorous, however protocol III of the 1980 Geneva Convention prohibits the use of incendiary weapons against civilian populations or by air attack against military forces that are located within concentrations of civilians. . Additionally, Article 1 of Protocol III of the Convention on Certain Conventional Weapons defines an incendiary weapon as “any weapon or munition which is primarily designed to set fire to objects or to cause burn injury to persons through the action of flame, heat, or combination thereof, produced by a chemical reaction of a substance delivered on the target”.
Use of these weapons against civilians or in civilian areas is forbidden, although many countries have reserved their rights to use them if they can justify the need.
More: